Standard chemotherapy and an autologous stem cell transplant proved ineffective, and his prognosis worsened following a severe Covid-19 infection. With traditional therapies failing and his disease progressing, doctors at Narayana Health City in September 2022, treated him with cutting-edge immunotherapy known as CAR T-cell therapy, initiated by IMMUNEEL in Bengaluru…reports Asian Lite News
A 42-year-old man from Bengaluru has successfully achieved “durable remission” from a relapsed cancer via CAR T-cell therapy, said doctors on Tuesday hailing it as a revolutionary cancer treatment.
The patient, Hamza Khan, was initially diagnosed with follicular lymphoma, a cancer of the lymph nodes, in 2020. His condition required multiple rounds of chemotherapy and maintenance with rituximab.
Despite the efforts, his cancer relapsed in February 2022, leaving him with few viable treatment options.
Standard chemotherapy and an autologous stem cell transplant proved ineffective, and his prognosis worsened following a severe Covid-19 infection. With traditional therapies failing and his disease progressing, doctors at Narayana Health City in September 2022, treated him with cutting-edge immunotherapy known as CAR T-cell therapy, initiated by IMMUNEEL in Bengaluru.
“CAR T-Cell therapy has revolutionised the landscape of cancer treatment, offering a lifeline to patients who have exhausted conventional options,” Dr Sharat Damodar, Senior Consultant Haematologist and Head of Adult BMT at Narayana Health City, told IANS.
“This groundbreaking success heralds a new era in cancer treatment, providing hope to countless patients facing relapsed cancers,” he added.
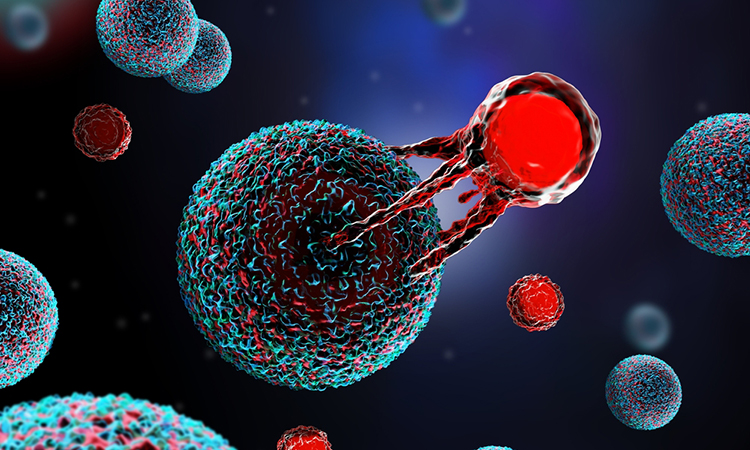
CAR T-cell therapy, or Chimeric Antigen Receptor T-cell therapy, is a groundbreaking form of immunotherapy used primarily to treat certain types of cancer, particularly blood cancers like leukaemia and lymphoma. The therapy involves modifying a patient’s T-cells, a type of white blood cell that plays a central role in the immune response, to better recognise and attack cancer cells.
“The advancements ensure that world-class treatment options are accessible within India. This has a lot of potential, as immunotherapy has fewer side effects and hence can be given to more people and uses the patient’s own cells,” Dr Damodar said.
“Hamza’s case exemplifies the potential of CAR T-Cell therapy to provide durable remission in relapsed lymphoma,” he said.
The therapy was approved for commercial use by the Central Drugs Standard Control Organisation (CDSCO) in October 2023.
NexCAR19, jointly developed by scientists at the Indian Institute of Technology, Bombay, and Tata Memorial Hospital in association with industry partner ImmunoACT, became the first CAR-T cell therapy to get approval in India.
“The manufacturing process is different for different varieties of CAR-T cells and this will change potency and efficacy. Long-term cure rates are also different,” Dr Damodar said.
“Cancer is just like bacteria and viruses. You get cancer when your body’s immune cells become dysfunctional. So in CAR T-cell therapy, we remove the immune cells from the patient and engineer them so that they become active against cancer again,” Dr Vipul Sheth, Senior Consultant, Hemato Oncology department at Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), told IANS.
He called the therapy a “living drug”. Dr Sheth said that “the one-time treatment fights against and eliminates cancer, and remains in the body”.
“So next time if there is an exposure to the cancer, it can get activated and fight on its own. Because of this treatment, you can treat many refractory patients. So basically those that do not even respond to stem cell transplant or chemotherapy, in those patients you can give CAR T cell therapy,” he said.
ALSO READ-Research Reveals Nightmares as Early Warning for Lupus Patients